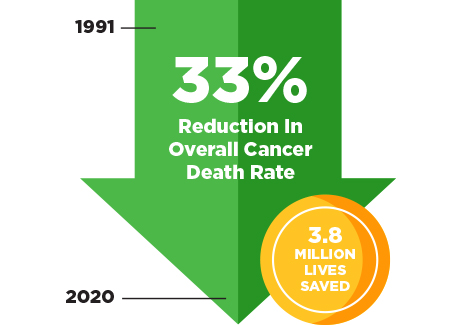
Learn about the cancer survivors featured in the 2023 edition of the AACR Cancer Progress Report.
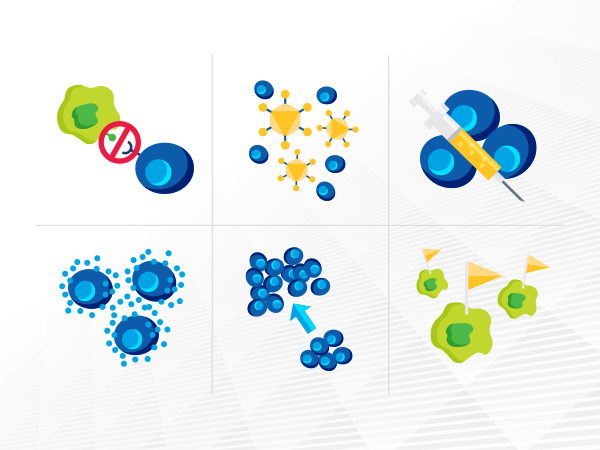
Cancer immunotherapeutics, which unleash a patient’s immune system to fight the disease, have emerged as one of the most exciting new treatment approaches.
Learn More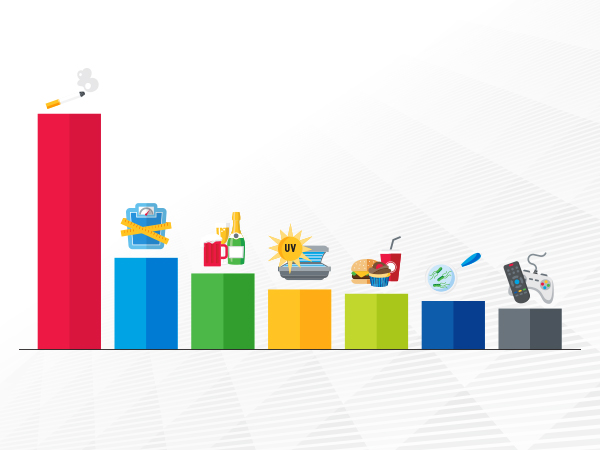
An estimated one in four cancer cases diagnosed each year in the United States is the result of potentially preventable risk factors.
Learn More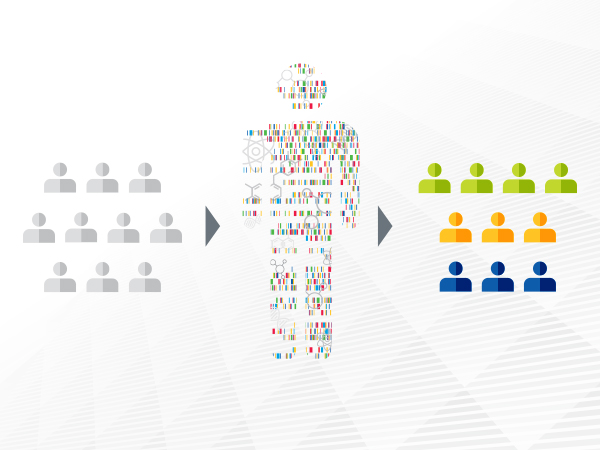
Advances in precision medicine are offering clinicians a new arsenal of molecularly targeted therapeutics against cancer.
Learn More